Targeting Plant Nutrition
Effective and Efficient Strategies for Lowering Inputs and Improving Production

The application of nutrients, or fertilisers, that plants require is an important component in primary production operations. For numerous reasons, crops or pastures, may not naturally get all the nutrients they need from the soil in order to perform well. The considered application of fertiliser is often necessary to meet the desired outcomes of enterprise programs.
However, applying nutrients is a costly business. The price of fertilisers continues to rise as global supplies are used up. A decline in soil health, as a result of agricultural practices, has led to a greater reliance on and requirement of fertiliser to maintain production. There are also major environmental issues associated with the run-off or volatilisation of commonly used fertilisers.
More than ever, there is both an economic and environmental imperative to make nutrient applications as efficient and effective as possible. It is important that growers understand how to determine soil and/or plant nutrient status with appropriate testing and make well informed decisions around fertiliser application in order to get good results and lower costs.
This article will explore how to:
- efficiently apply what’s needed to meet plant requirements at different stages of the season or in different conditions.
- reduce the amount of energy plants have to spend accessing, acquiring and assembling nutritional amendments.
- enhance the uptake and reduce the loss of applied nutrients.
- avoid decommissioning and/or damaging soil life with fertiliser applications.

Plant Nutrition
Essential Plant Nutrients
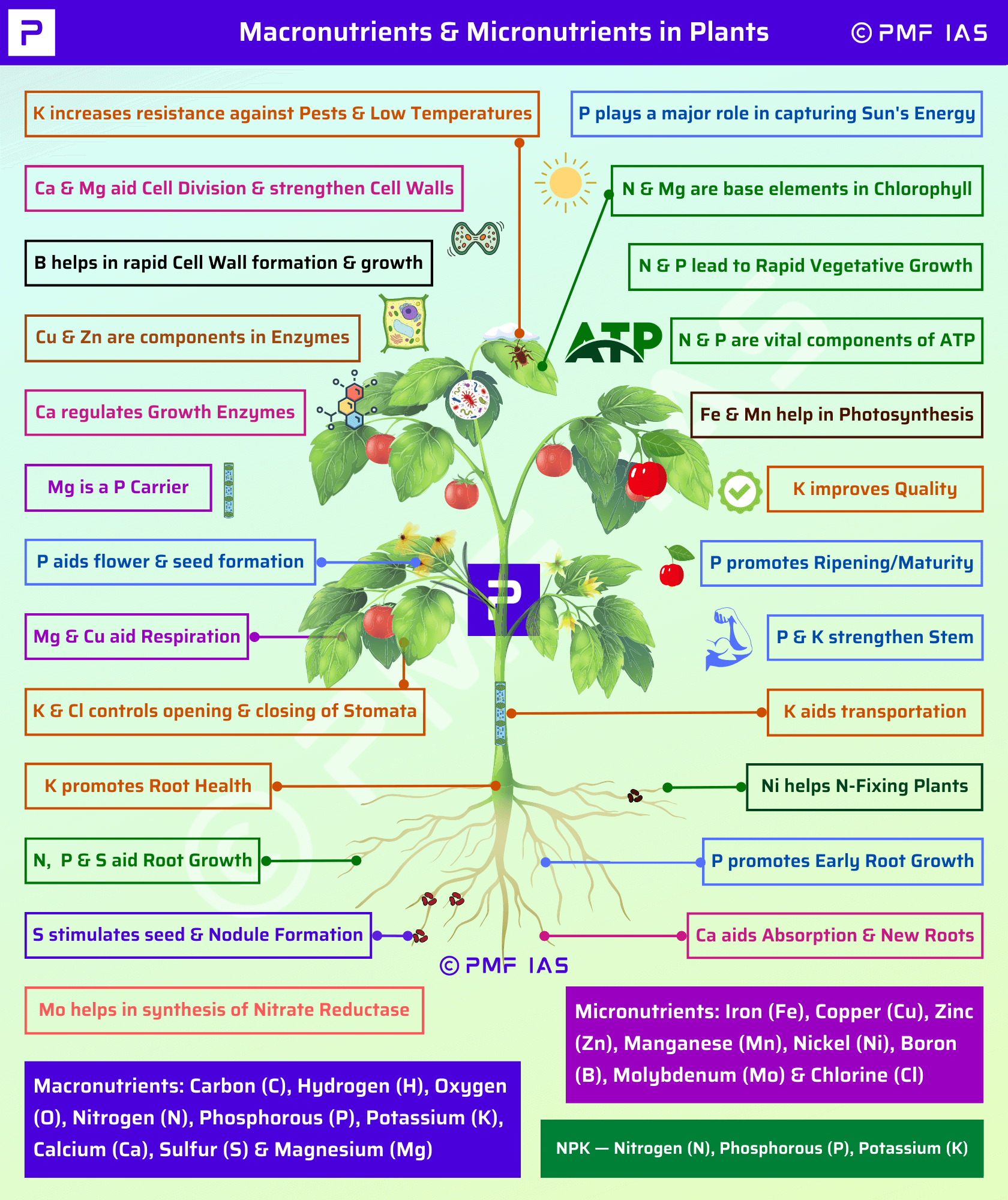
Plants require various amounts of essential nutrients in order to grow, function and reproduce.
These elements are deemed essential because many plants require all of them, to some degree, in order to complete their lifecycle.
|
Macronutrients are required in larger amounts & include: |
Micronutrients are required in small amounts & include: |
|
Nitrogen |
Zinc |
|
Potassium |
Copper |
|
Calcium |
Molybdenum |
|
Magnesium |
Chlorine |
|
Phosphorous |
Boron |
|
Sulphur |
Iron |
|
Manganese |
|
Plant Nutrient Uptake
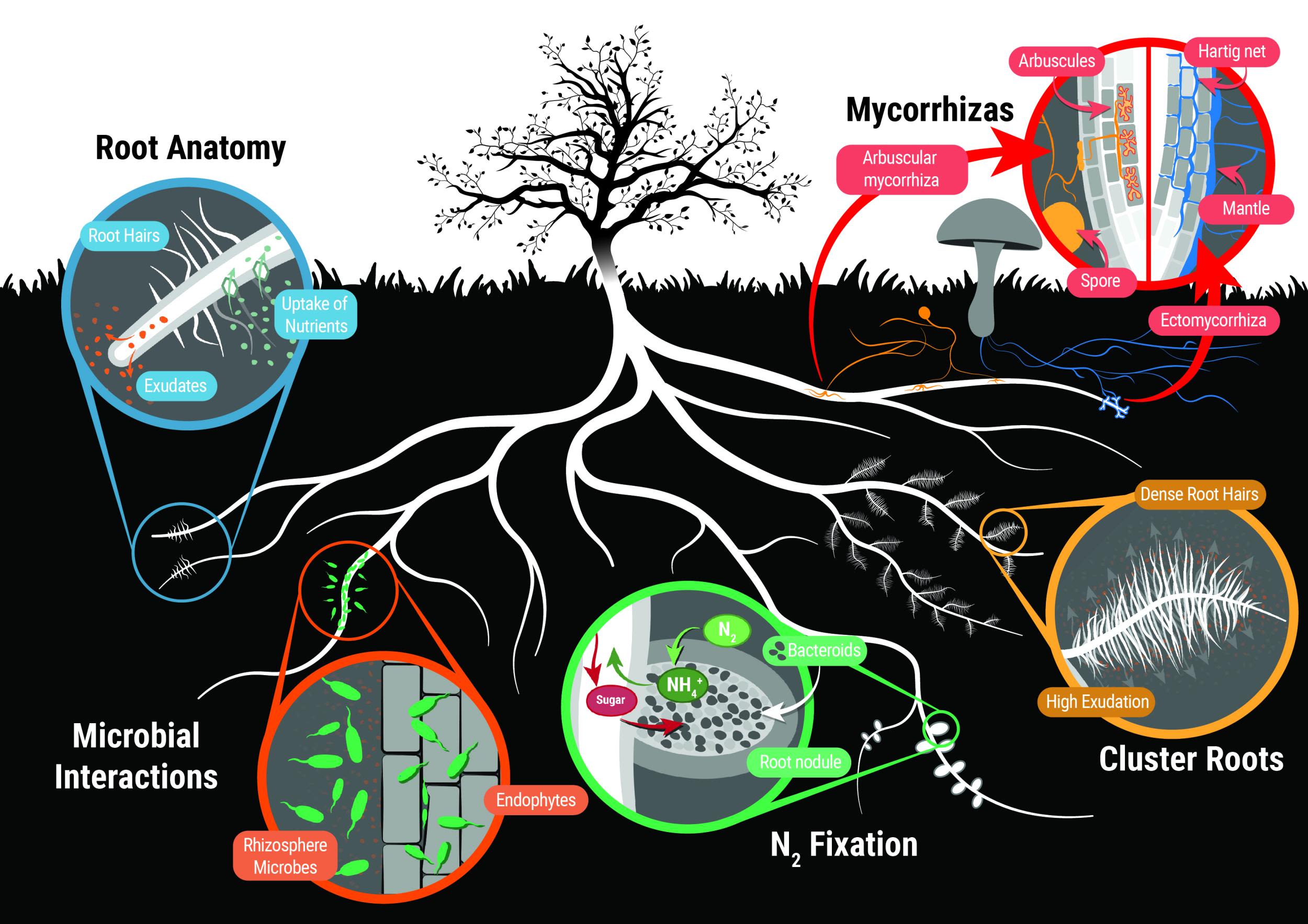
Different nutrient acquisition strategies. (Source: Plants in Action)
Different nutrient acquisition strategies. (Source: Plants in Action)
Plants take up most of the nutrients they need through their roots from the soil environment.
- They acquire these nutrients in varying degrees from the soil solution, clay/humus exchange sites, soil biology, the breakdown of organic materials and the surfaces of soil mineral particles.
- It used to be thought that plants only take up ionic nutrients in solution but it is now known that plants can take up whole molecules, complete compounds like amino acids and proteins and even bacterial cells.
- Plants can also absorb a small amount of nutrients from gases, solutions, organic sources and dusts above the ground, through their leaves, stems and reproductive organs.
Applied Nutrition
The application of nutrients has the potential to significantly improve, but can also adversely affect, production outcomes, depending on the context, type of fertilisers, amounts used and methods of application. It is important that growers understand how to determine soil and/or plant nutrient status with appropriate methods and make well informed decisions around fertiliser application in order to get desired results, lower costs and prevent ecological issues.
|
Nutrient Use Efficiency in Agricultural Ecosystems |
|
|---|---|
|
Various environmental factors affect NUE such as: • soil dynamics (exchange capacity, moisture content, leaching/runoff, fixation etc…) • plant characteristics (nutrient absorbing capacity, age, cultivars, root morphology etc…) • climate (sunlight, rainfall, wind etc…) • nutrient application (amount, form, timing, placement etc…) • nutrient application (amount, form, timing, placement etc…) |
|
|
Nutrient |
Efficiency (%) |
|
Nitrogen |
30–50 |
|
Phosphorus |
15–20 |
|
Potassium |
50–60 |
|
Sulphur |
8–12 |
|
Zinc |
2–5 |
|
Iron |
1–2 |
|
Copper |
1–2 |
|
Manganese |
1–2 |
|
Boron |
2–3 |
|
Molybdenum |
2–5 |
Soil Nutrient Status & Testing
There are a range of methods that can be used to determine the status of your soil or plants to inform the decision-making process around nutrient applications that can be made to improve plant nutrition and minimise waste.
Soil Nutrient Bank Accounts
At any one time there are pools of soil nutrients:
- dissolved in the soil solution
- held on exchangeable sites
- within soil organisms
- within organic matter
- locked up in the soil mineral matrix
These pools of nutrients are in a state of flux. Nutrients are constantly being removed from the soil solution and exchange sites by plants, released from organic matter and liberated from the soil mineral matrix by soil microbes and so on.
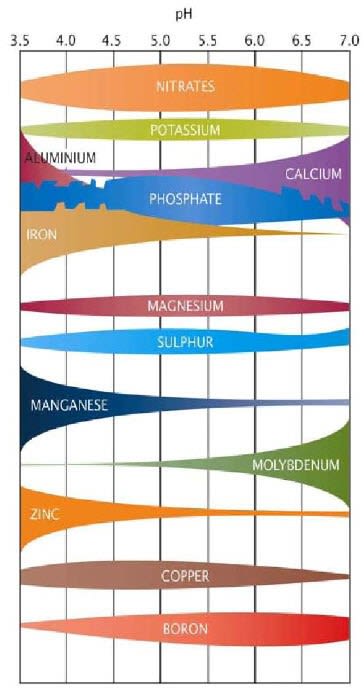
Variables such as soil moisture, temperature, cation exchange capacity (CEC), phosphorous buffering index (PBI), acidity/alkalinity (pH) redox state (Eh) and soil carbon levels also significantly influence the state and place nutrients occupy in the soil and their plant availability.
Soil testing methodologies are designed to identify significant soil characteristics and the levels of essential nutrients in the different soil fractions. These are then interpreted in relation to plant nutrient availability.
Field Assessment of Soil
Soil characteristics such as pH, moisture, colour, texture and structure, can be visually observed or tested in the field but these only provide general indication of soil fertility.
To obtain more comprehensive information, soil samples can be sent to certified laboratories that offer test packages that cover a range of different parameters.
Available nutrient test methods use solutions, made up of components such as ammonium acetate, sodium hydrogen carbonate, sodium bicarbonate and water in various concentrations, to extract nutrients from the soil medium. They are designed to give you an idea of what nutrients are available for ionic uptake from the soil solution or exchange sites at that point in time, but don’t measure nutrients held in the organic fraction, biology and the soil mineral matrix.
The Phosphorus Buffering Index (PBI), which provides an indication of a soils tendency to bind phosphorous, is a good example of a parameter used to account for such variables.
There can be nuances in the extraction methods that different labs use to determine nutrient availability so it is important that reference levels used are aligned with the chosen extraction methods.
There can be variability in the extraction methods different labs use to determine nutrient availability so it is important that reference levels used are aligned with the chosen extraction methods.
Some laboratories also conduct a total nutrient soil test that is designed to determine the quantities of nutrients in other fractions of the soil, such as organic matter, biology and soil particles, along with the nutrients held on exchange sites and dissolved in the soil solution. This test uses highly acidic solutions of hydrochloric and nitric acid to extract nutrients from the soil medium.
While the nutrients recorded are not all plant available, it gives an indication of total nutrient quantities in the soil bank that may also become more or less available over time with weathering, biological activity and changing seasonal conditions.
Things to consider when collecting soil samples for testing.
- Accounting for isolating different soils
- Depth of sampling
- Accurate cross section/representation
- Avoiding sites of potential contamination
- Adequate volume of soil
- Seasonal conditions
- Handling
- Storage
It is best to follow the sample collection guidelines of the laboratory you are sending your soil samples to.



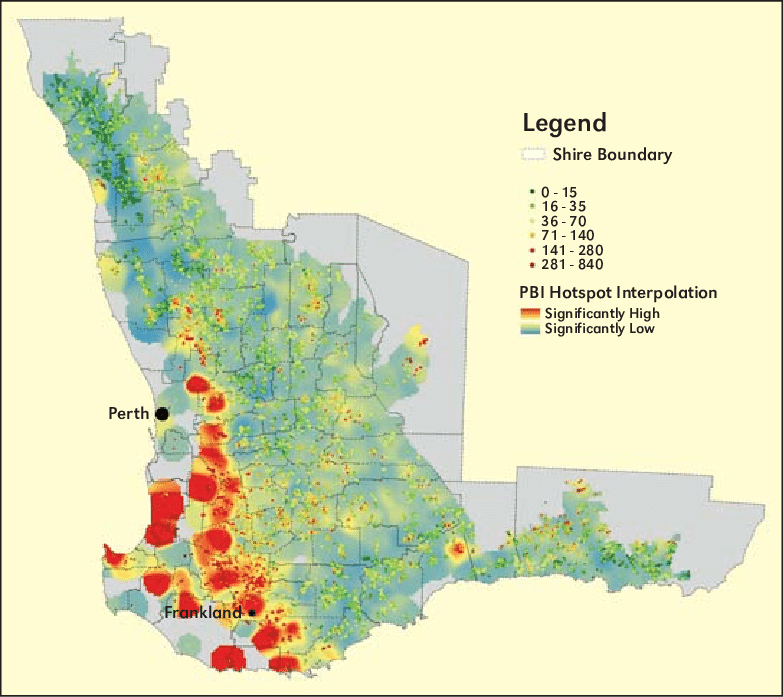
The phosphorus buffer index (PBI) map of Western Australia, grouped according to the national PBI categories, highlights locations of soils with PBI's above 70. These are marked in orange (10% of total samples) and red colors (5% of total samples) and can be described as mainly gravelly forest soils.
The phosphorus buffer index (PBI) map of Western Australia, grouped according to the national PBI categories, highlights locations of soils with PBI's above 70. These are marked in orange (10% of total samples) and red colors (5% of total samples) and can be described as mainly gravelly forest soils.

Sample Collection Guidelines
How to soil sample for the Healthy Estuaries DIY sampling program
Identifying hazards and planning your transect for Healthy Estuaries assisted soil sampling.
Accurate Soil Sampling Guide
A Guide for 'fit for purpose' sampling
Plant Nutrient Status & Testing
The optimal concentration range for the different nutrients within plants varies with species, parts, stages of growth and growing conditions.
The balance between different nutrients and their interactions within plants also has a bearing on growth. Nutrients are translocated via the xylem stream, from roots to shoots. Along the way they may be deposited in roots, stems and leaves or transferred to phloem sap. Some nutrients are more phloem mobile than others and can be readily partitioned to different plant parts as needed or stored.
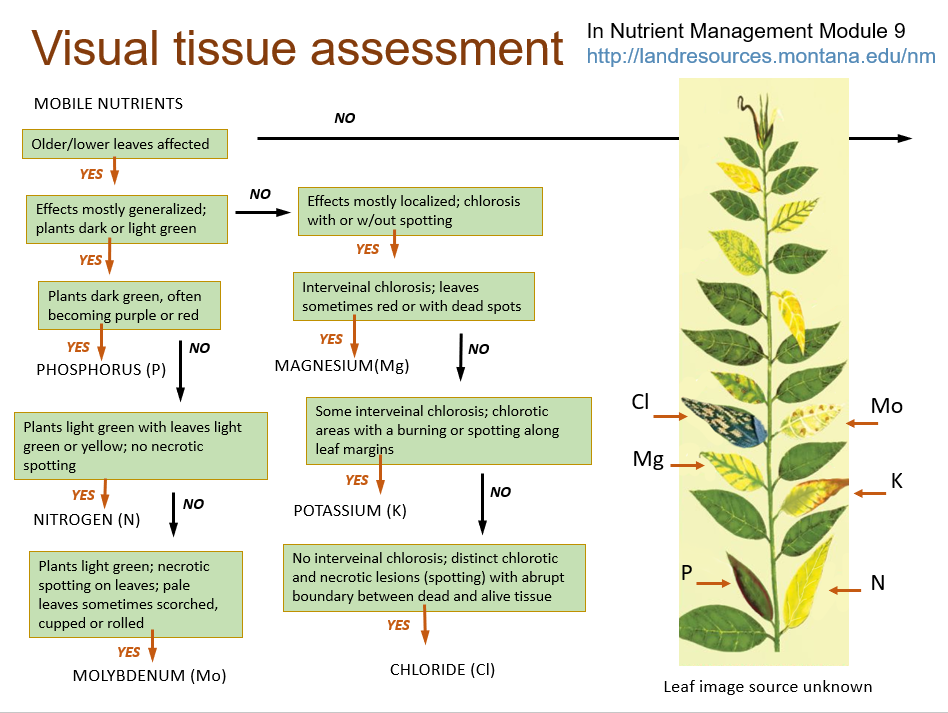
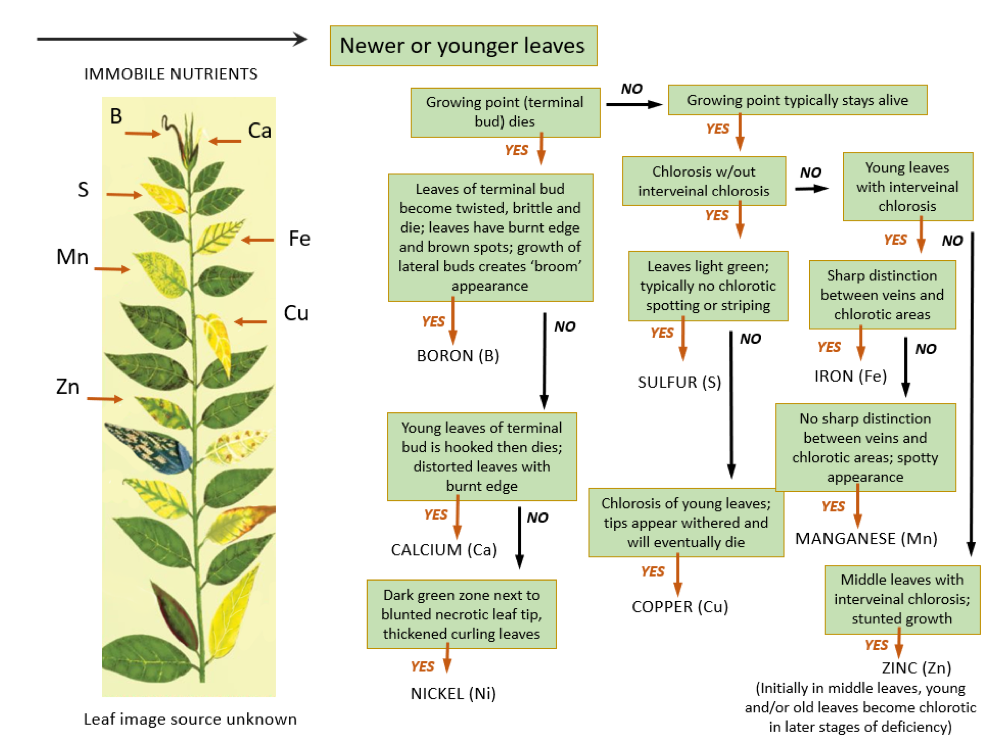
Field Assessment of Plant Nutritional Status
A range of plant testing methodologies can be used to identify the levels of nutrients and other characteristics such as sugar content, pH and electrical conductivity in different plant parts.
Handheld Meters

Refractometers:
- Refractometers measure brix which is a measurement of dissolved solids i.e. sugars and ionic nutrients
- High brix levels are an indication of high sugar production in combination with nutrient density
- A fuzzy line in the brix reading is indicative of nutrient density, particularly calcium
- The brix readings can vary with different weather conditions and times of day
- Brix levels should rise through the day and drop towards the evening. If this doesn’t happen, there may be a boron deficiency
- High nitrate levels can lead to increased water uptake and diluted brix readings

Horiba LAQUAtwin Meters
These are handheld meters that can be used in the field to monitor: pH | EC | Potassium | Calcium | Nitrate | Sodium
These meters were not designed specifically for plant testing so established reference levels for different plant parts, species, stages of growth aren’t always available.
However, with some understanding of the parameters you’re testing, they can still be used to gain valuable information, track crop health over time and carry out side by side comparisons.
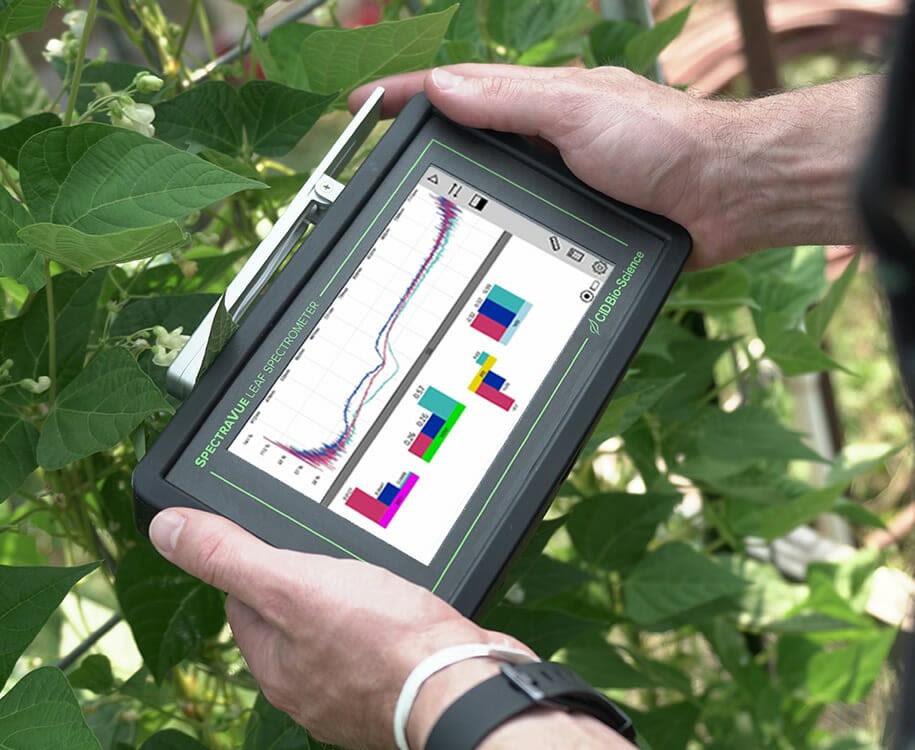
Spectrometers
Spectroscopy is a newly emerging technology that can be used to analyse the nutrient status and other parameters of plants in the field, in real time. While spectrometers are not currently readily accessible to growers, they are developing fast and look to be a promising tool for plant analysis in the near future.
Leaf Tissue Testing

Taking Leaf Tissue Samples
Taking Leaf Tissue Samples
In tissue tests, the ash of whole leaves is analysed for nutrient content.
There are well established leaf tissue reference levels for most commercial plant species at different stages of growth.
All nutrients in the tissue and in the sap are recorded. One issue with this is that nutrient deposits in the tissue from earlier growth may not correlate with the more immediate supply of nutrients, so there can be a lag of several weeks before deficiencies/toxicities show.
As leaf tissue samples are burnt, the state that different nutrients are in and characteristics such as sugar content, pH and EC are not reported.
Laboratory Plant Testing
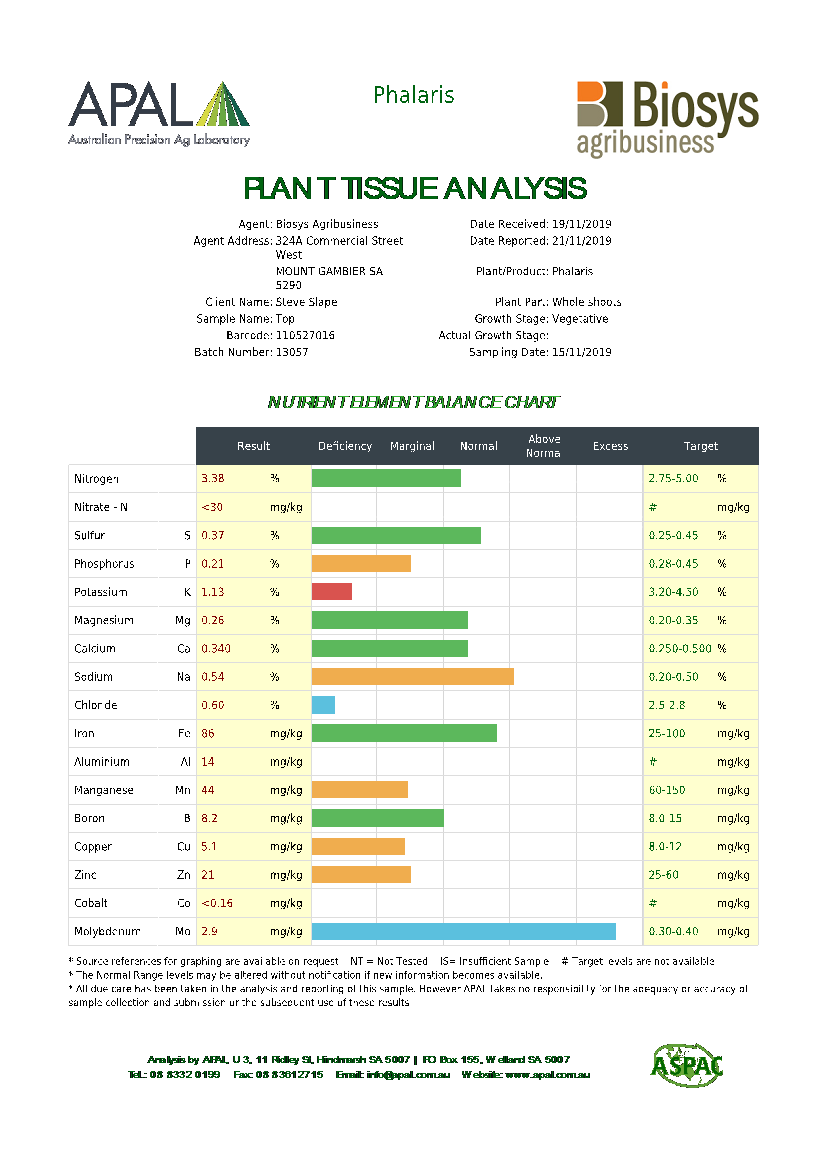
For a more detailed breakdown of plant nutritional status etc, it is common practice to send plant samples to a laboratory for tissue or sap analysis.
Sap Analysis
Plant sap analysis can be carried out to get a reading of different sap parameters at the time of sampling. It gives a fairly good indication of current nutrient supply, so issues can be picked up and addressed more immediately. The ionic state of nutrients levels, sugar levels, pH and EC can also be recorded. As the tissue from the sample is discarded, nutrient sap levels are less influenced by stage of growth and processes such as lignification etc…
Plant sap analysis hasn’t been around as long as tissue testing so reference levels for commercial crop species aren’t as well established.
Most commonly, the first fully developed young leaves or petioles samples are used for sap analysis. It is important to keep in mind that petioles are a pipeline for water, nutrient and sugars, and that there may be some fluctuations in readings, over a given period of time and under different conditions, based on how plants are allocating mobile fractions.
The same consideration needs to be given to the fact that plants prioritise new growth when partitioning mobile nutrients, and the sampling of young leaves alone, may not pick up deficiencies/excesses that may show in older leaves. Some laboratories recommend differential leaf sap analysis where both young and older leaf samples are taken to account for and track mobile nutrient partitioning.
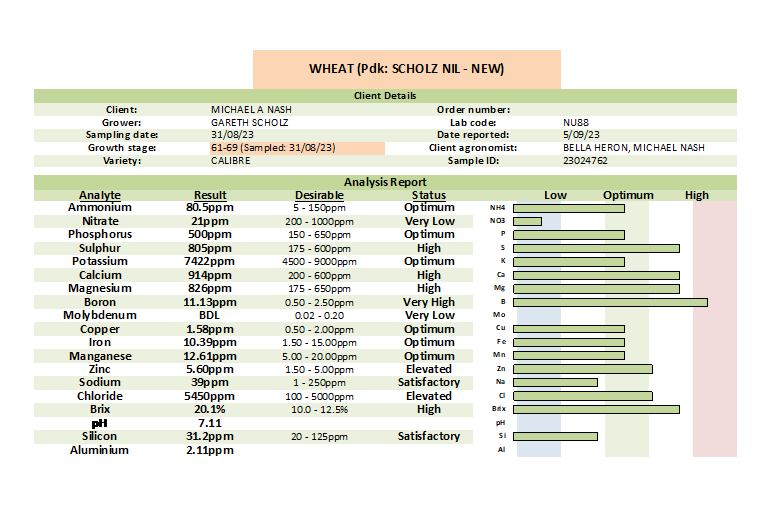
Petiole Sap Analysis
Petiole Sap Analysis
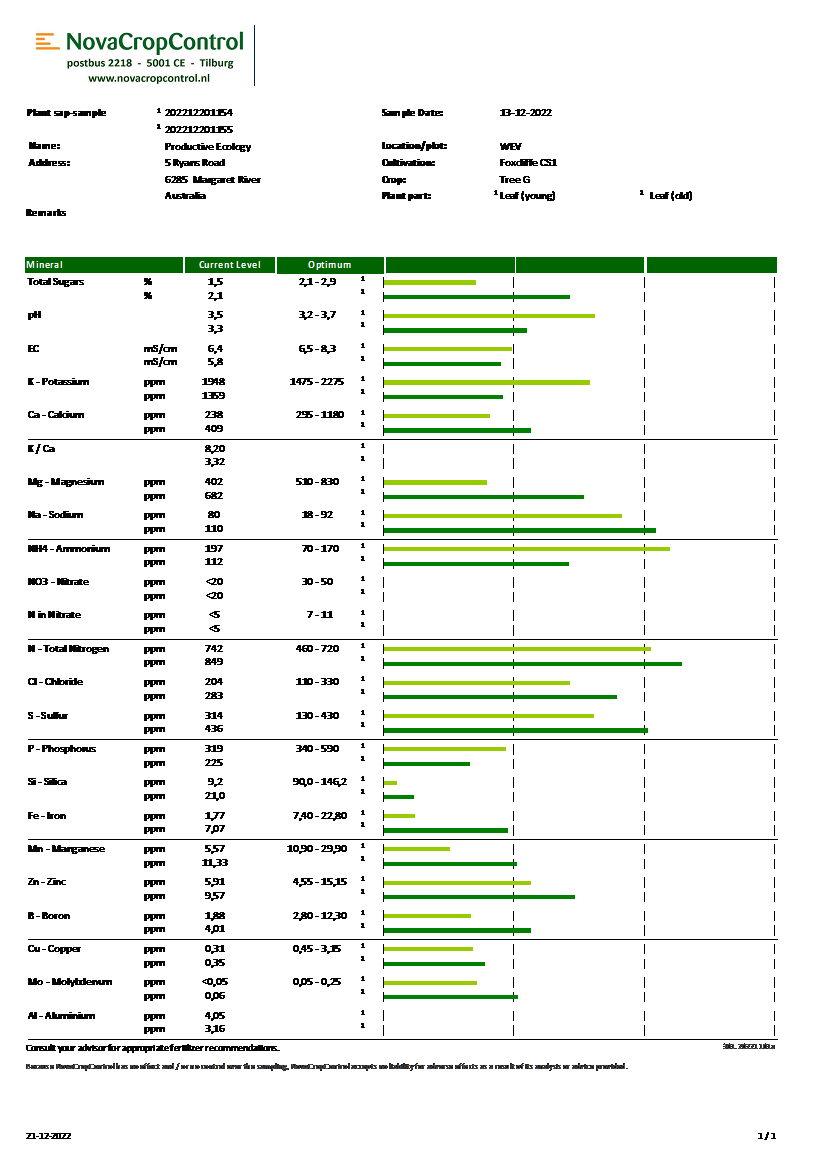
Differential Leaf Sap Analysis
Differential Leaf Sap Analysis
Things to consider when collecting plant material samples:
- What plant parts you are testing
- Accurate cross section/representation
- Adequate volume of material
- Weather conditions
- Time of day
- Handling
- Storage
When sending samples away for analysis, it is best to follow the sample collection guidelines the laboratory you are sending your samples to.

Fertilisers
Nutrients naturally occur in varying quantities within growing environments as inorganic gases, solutions and minerals, and the organic compounds of life such as plant material, animal bodies and microbial mass.
A fertiliser is a natural or synthesised substance containing chemical elements that is manufactured, represented, sold or used as a means to improve the growth and productiveness of plants
Inorganic nutrients are used for the production of synthetic fertilisers, and organic nutrients are derived from organic materials

Common Synthetic Fertilisers
- Urea
- Sulphate of Ammoni
- Mono Ammonium Phosphate (MAP)
- SuperPhosphate
- Calcium Nitrate
- Sulphate of Potash (SoP)
- Mono Potassium Phosphate (MKP)
- Magnesium Sulphate
- Zinc Sulphate
- Solubor
Common Organic Fertilisers
- Manure based products
- Fish hydrolysates
- Blood and bone
- Seaweed products
- Compost
Note: Products like Lime, Gypsum, Dolomite, Rock Minerals, Sulphate of Potash, Magnesium Sulphate, Zinc Sulphate, Solubor, Sea Minerals etc… can be used as inputs in organic systems but strictly speaking, they are inorganic fertilisers
Fertiliser Grade: An expression referring to the guaranteed percentage of available Nitrogen, Phosphorous and Potassium (N:P:K) by weight in a fertilizer
This Department of Primary Industries & Regional Development Fertiliser Calculator compares the nutrient content of more than 1500 commercially-available fertilisers.
Use this Nutrient Calculator for high rainfall pastures in Western Australia developed by DPIRD, to evaluate your soil sample results, and to estimate soil nutrient status and requirements, and the fertiliser quantities to supply those nutrients. Find out more about the calculator.
Fertiliser Fast Facts:
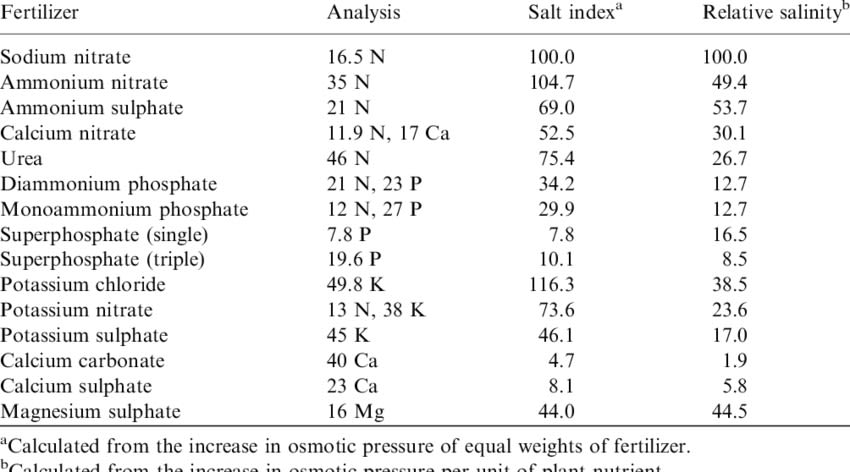
Solubility
Not all fertilisers are soluble in water. The following rules of thumb are useful for determining the solubility of fertilisers, although there may be exceptions.
- All ammonium, nitrate, potassium, sodium and chloride salts are soluble
- All oxides, hydroxides and carbonates are insoluble
- All sulfates are soluble except for calcium sulfate
Form
Fertiliser products can be applied in dry or liquid form.
Dry applied fertilisers: Dry granules | Pellets | Coarse minerals | Rock dusts
Dry applied fertilisers can be broadcast, banded near crops or placed in planting furrows.
|
Pros |
Cons |
|---|---|
|
Generally cheaper |
Poorer distribution through the soil profile |
|
Store well |
Harder to apply evenly |
|
Less applications required |
Slower nutrient uptake |
|
Can be applied in a wide range of conditions |
Granular fertilisers break-down in damp environments |
|
Better suited for heavy pre-planting applications |
Need moisture to break down so not as effective in drier weather |
|
Slow-release options |
|
|
Less compatibility issues |
Fertilisers applied as liquids: Soluble powders | Microfine suspension grade products
Liquid fertilisers can be applied as a soil drench, injected/dripped into planting furrows, through fertigation or in foliar sprays.
|
Pros |
Cons |
|---|---|
|
Can be applied to the soil and the plant |
Must be applied more frequently |
|
Can be put out with fertigation and spray units |
May separate, or settle out when stored. |
|
Better distribution over and through through the soil profile |
Set-up or application processes may be expensive |
|
Fast acting |
Ready-made liquid products are often shorter lived than dry fertilisers |
|
Can be effective in dry conditions |
Can’t get as much out |
|
Easy to blend and apply with compatible products |
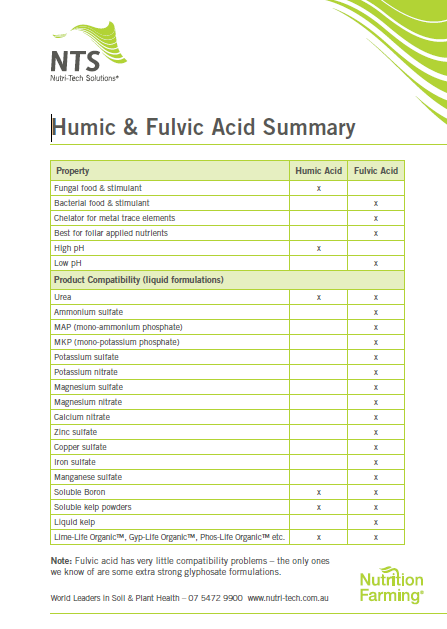
Non-Nutrient Fertiliser Components: These are designed to stabilize and/or buffer applied nutrients, adjust the pH, enhance nutrient uptake, stimulate biological activity.
Fertilisers products may contain:
- binders
- coatings
- chelating/complexing agents
- acidifying or alkalising agents
- humic substances
- biological stimulants/foods








Fertiliser Application Efficiency
Soil Nutrient Application
Soil chemical, physical and biological characteristics and weather conditions all have a major bearing on the plant availability, fixation, leaching and cycling of soil applied nutrients. Some nutrients are also more prone to getting locked up and less mobile in the soil whilst others are less stable and susceptible to leaching.
It is important that we know our particular soil characteristics and seasonal weather conditions and the impact of these in relation to the fertilisers we apply.
Consideration needs to be given to how to best apply the nutrients we need in our context.
Some examples of how we would do this include:
- Only applying soluble fertilisers in suitable conditions, when seasonal weather is less extreme
- Using the right form of nutrients for the conditions (i.e. not applying dry fertilisers in dry conditions)
- Negotiating a favourable balance of Carbon to Nitrogen in the soil to ensure the biology has both a source of energy and the nitrogen to make proteins.
- Targeting the placement of soil applications, especially immobile nutrients such as phosphorous, for better plant uptake minimal waste
- Timing applications to foster delivery of the required nutrients to plants when they need them
- Applying soluble fertilisers in lower amounts, more regularly or using slow release forms.
- Accounting for the poor uptake some soil applied nutrients (i.e. the trace elements)
Foliar Applied Nutrition
The application of nutrients to the foliage of plants can also be an effective way to improve crop nutritional status. There are several advantages to the foliar application of nutrients.
It offers:
- a means to get nutrients into the plant when soil delivery is compromised by adverse conditions
- efficient uptake of applied nutrients, so significantly lower quantities are needed
- rapid plant uptake to immediately address plant nutrient shortages, especially in leaves
- an effective way of getting trace elements into plants
The constraints of foliar applications are that they:
- have be applied more regularly
- can’t supply adequate quantities of macro nutrients
- require the equipment to get them out
- involve post germination traffic
- don’t deliver plant immobile nutrients, that may be needed, to other parts of the plant besides the foliage i.e. the root zone
- don’t effectively supply nutrients that may be required in the soil environment
|
Nutrient Mobility in Soil & Plants |
||
|---|---|---|
|
Nutrient mobility |
in the soil |
in plants |
|
Very Mobile |
Prone to leaching - nitrate Nitrogen, sulfate Sulfur, Boron |
Nitrogen, Phosphorus, Potassium, Magnesium (Deficiency symptoms appear first in older leaves and quickly spread throughout the plant) |
|
Moderately Mobile |
Ammonium Nitrogen (Ammonium Nitrogen is temporarily immobile), Potassium, Calcium, Magnesium, Molybdenum |
Sulfur, Copper, Iron, Manganese, Molybdenum, Zinc (Deficiency symptoms first appear in new growth but do not readily translocate to old growth) |
|
Immobile |
Organic Nitrogen, Phosphorus, Copper, Iron, Manganese, Zinc (Chelated forms of Copper, Iron, Manganese and Zinc are mobile and resistant to leaching) |
Boron, Calcium (Calcium is very immobile) |
|
Source: Growth and Mineral Nutrition of Field Crops, Third Edition. By Nand Kumar Fageria, Virupax C. Baligar, Charles Allan Jones, 2011. CRC Press, Taylor and Francis Group. |
||


Metabolic Shortcuts
Acquiring nutrients and assembling them into useful compounds takes energy. Ideally, we want to minimise the amount of energy plants have to spend doing this so that they have more energy to put towards healthy growth and production.
Measures that can be used to improve energy efficiency include:
- Ensuring that there is a good supply of nutrients in the root zone of our crops
- Maintaining a constant supply of available and exchangeable nutrients as these are easier for plants to acquire than those held within the soil mineral complex and organic materials
- Using organically chelated/complexed fertilisers to provide plant nutrients with pre-assembled molecules, saving them from having to build organic compounds from scratch
- Avoid applying large amounts of soluble nutrients at one time it takes more energy for plants to regulate uptake when there are excess concentrations of dissolved ions in the soil solution
- Improve soil conditions to avoid situations that affect nutrient availability i.e. high/low pH, reduced/oxidized redox states, high/low moisture levels, humus levels, biological activity.
As nitrogen is the nutrient that plants take up most from the soil it warrants special mention.
It takes lots of energy to convert nitrate to ammonia to the amino acids and proteins plants need. There must be an adequate supply of carbohydrates to fuel these reactions. Certain nutrients are needed to make the enzymes involved in these conversion processes (i.e. Sulphur and Molybdenum are part of the nitrate reductase enzyme, Nickel is part of the Urease enzyme). Other nutrients besides nitrogen are needed to assemble the full spectrum of necessary proteins i.e. Sulphur to form Methionine & Cysteine.
We can save plants lots of energy by using less energy costly forms of nitrogen, providing an additional source of energy i.e. molasses, and ensuring that there is an adequate supply of the other nutrients required for efficient nitrogen conversion and protein formation.
In this important and topical webinar, agroecologist Joel Williams of Integrated Soils introduces participants to the fundamentals of how nitrogen works in the nutrient cycle and what steps farmers can take to reduce their reliance on inorganic nitrogen through good nitrogen management for more resilient and profitable grazing systems.
In this episode of Talkin’ After Hours Jo & Kate chat to Joel Williams on the hot topic of Nitrogen – how it works in the nutrient cycle and how can we best optimise its use – particularly in grazing systems. The podcast summarises much of the information presented in the LCDC's earlier webinar with Joel on the same topic. Tune into the full podcast below.
Fertiliser Recipes
Foliar Fertilisers
Liquid Fertiliser Recipes
Acidic Liquid Trace Element Mix
Alkaline Humic Liquid Mix
Biologically Friendly Fertilisation
As soil biology largely determine structural properties and the availability, delivery and cycling of nutrients, we obviously want to minimize compromising and even promote biological activity with nutrient applications.
Things that can be done:
- Avoid burning soil microbiology with large applications of high salt fertilisers.
- Buffer high salt fertilisers with humic substances to reduce harming biology.
- Combine nutrients with biological foods, bio-stimulants, humic/fluvic compounds and chelating/complexing agents to encourage biological uptake
- Don’t place high analysis, soluble fertilisers near germinating seeds and fresh roots, especially Nitrogen & Phosphorous, as they decommission biology
- Consider substituting in furrow, planting applications of soluble fertilisers with mineral, organic, chelated/complexed fertilisers and/or foliar applications during establishment
- Keep in mind, fertilization can have a positive effect on soil microbiology where soil is poor by improving plant growth which translates to more microbial food. Microbes also need nutrients
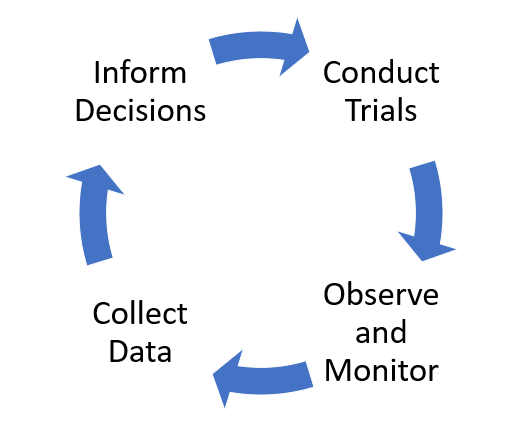
Trial and Observation
When introducing any new practice, it’s wise to try things on a small area first to get an idea of what’s involved and well they work.
Side by side trials and analysis provide valuable information on the costs and benefit of fertiliser strategies. Once we are confident of the merit of certain fertiliser applications and familiar with their implementation, we can adopt them on a wider basis.

This document is a downloadable summary of this online article & content hub ‘Targeting Plant Nutrition’.
This article was produced by 'Talkin' After Hours', the Lower Blackwood Landcare's Online Community & Information Hub
The article was written & collated by Mark Tupman from Productive Ecology to assist land managers in making making informed decisions around the use of fertilisers for soil and plant nutrition.

The development of this article was funded through Soil Wise. Soil Wise is funded by the National Landcare Program Smart Farms Small Grants – an Australian Government initiative. It is supported by Healthy Estuaries WA – a State Government program.

©Talkin' After Hours. This article cannot be reproduced without the permission of the Lower Blackwood LCDC